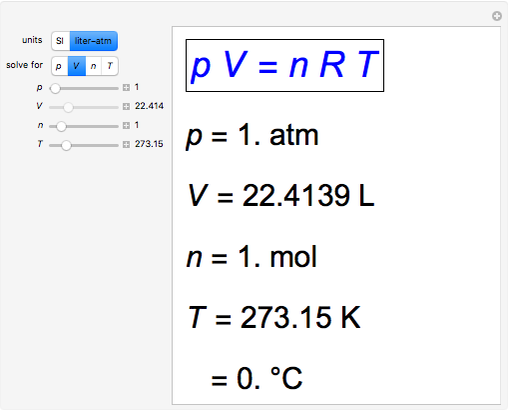
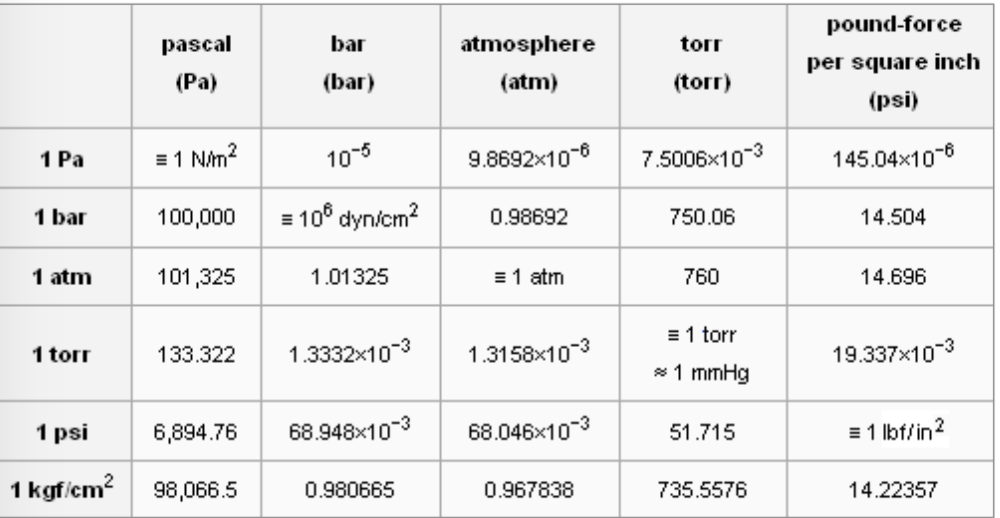
SOLVED: 1 atm = 760 mm Hg 760 torr = 1 atm 101325 Pa = 1 atm 101.3 J = 1 L atm 1 nm = 1 x 10^-9 m 0.08212 L

Is this incorrect? Standard Condition = 298K, 1 ATM and 1 M, STP = 273K, 1 ATM, 22.4 L. Shouldn't the card say Standard Condition is 298K? : r/Mcat

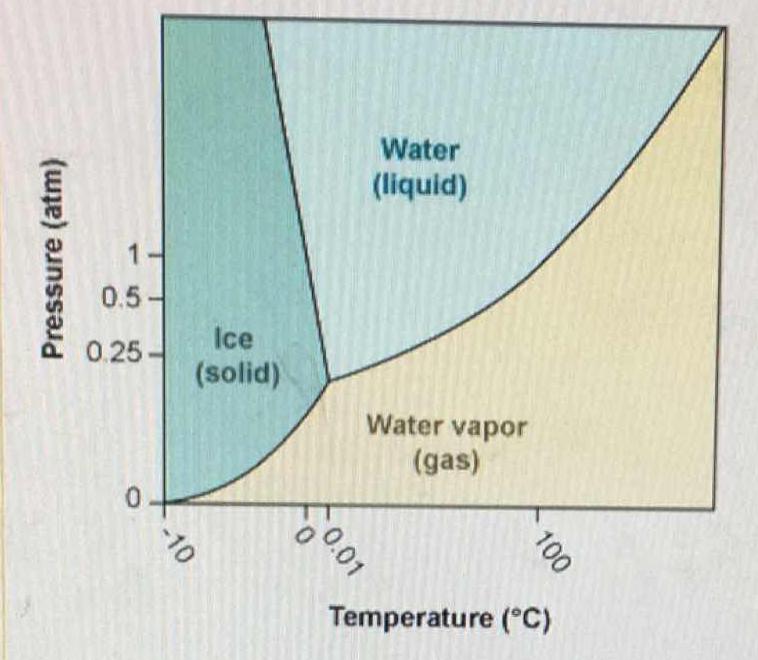
boiling point - Is it correct to say that SOME iodine undergoes sublimation at 1 ATM - Chemistry Stack Exchange

pressure, conversion units into defferent units,atm,bar,torr,psi,Pascal,mmHg, numerical,and examples - YouTube

Gas Laws Chapter 5. Pressure Force per unit area Measured in Atmospheres ( atm) Mm of Hg = Torr Pascals or kiloPascals (Pa or kPa) - ppt download

At 1 atm and 273 K the density of gas, whose molecular weight is 45, is: (a) 44.8 g/L (b) 11.4 g/L (c) 2 g/L () 3 g/L ise from the